Physics: Viewer's Request: Thermodynamics #3: Why Do We Use (delta)U=Q-W and (delta)U=Q+W ? - YouTube

https://www.grc.nasa.gov/www/Wright/airplane/Images/thermo1f.gif | Energy research, Internal energy, Thermodynamics
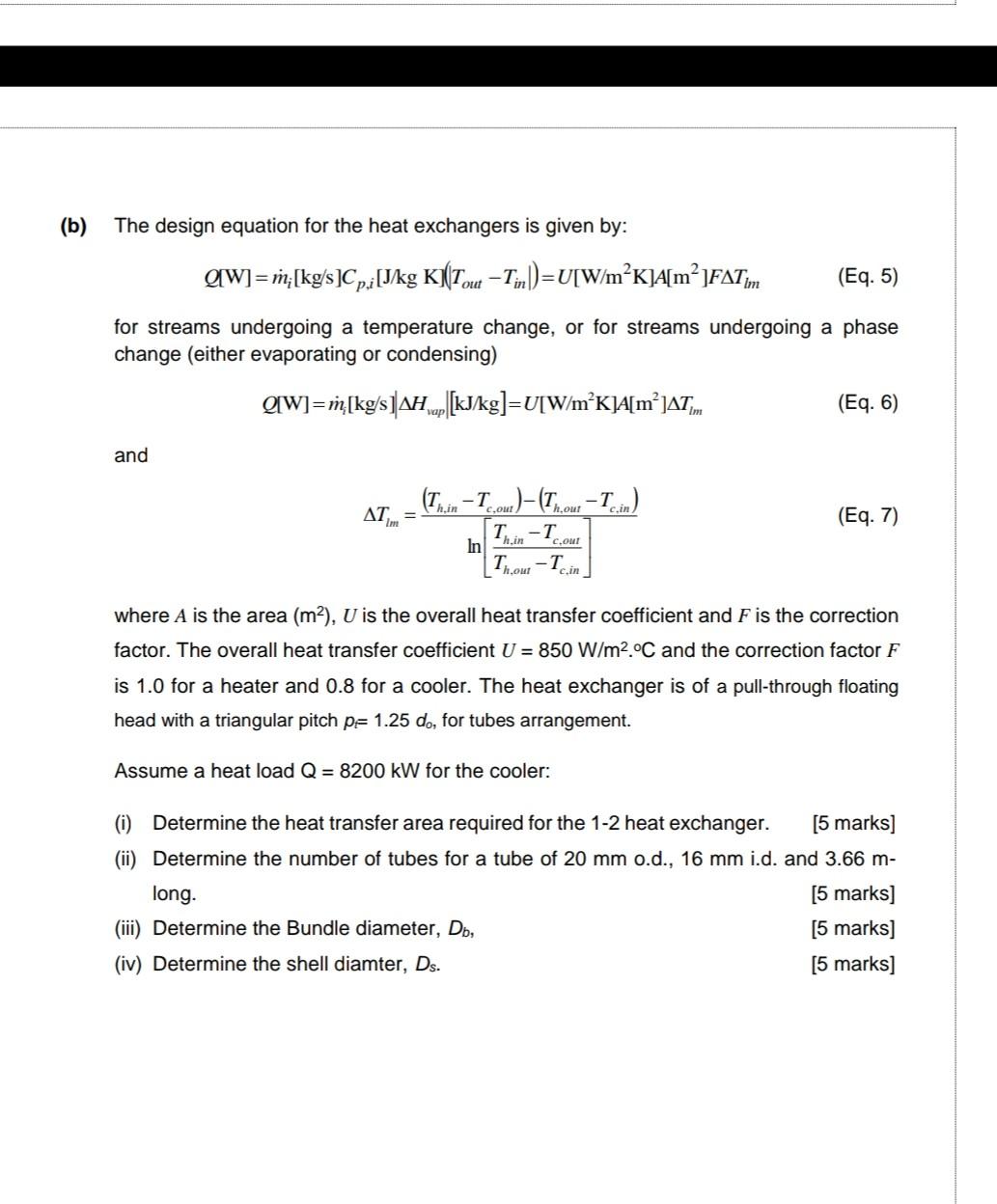
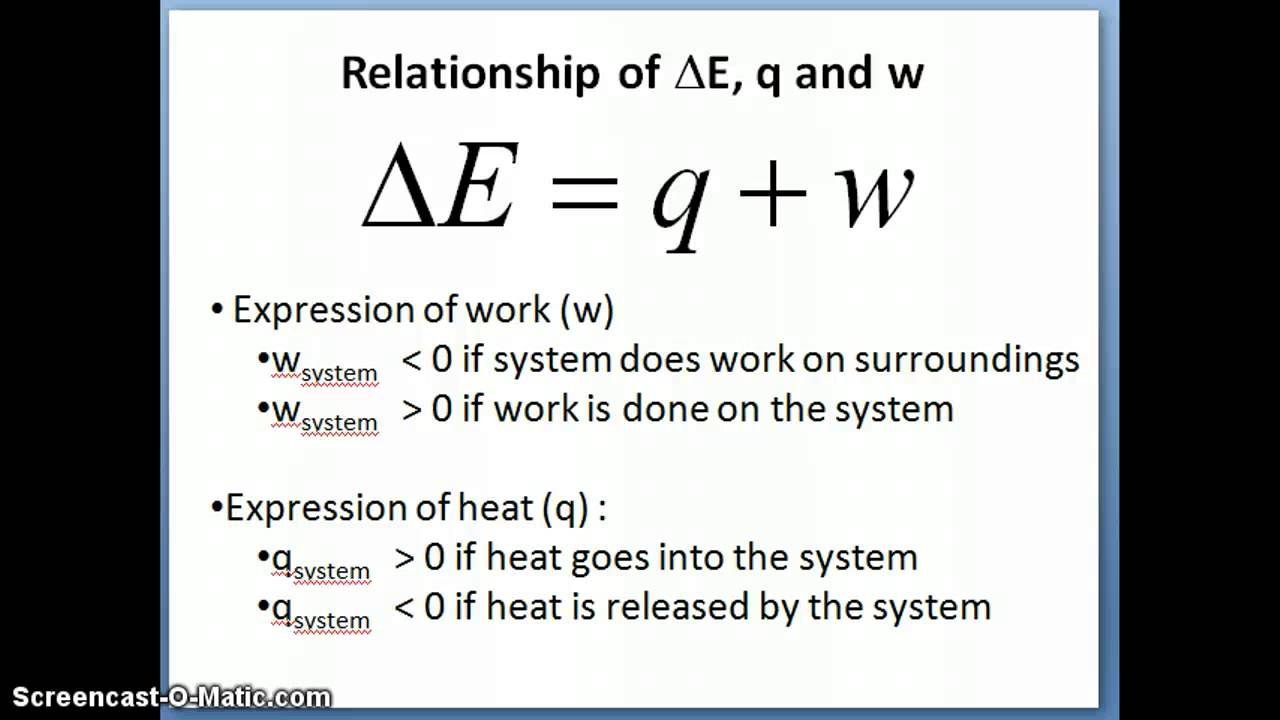
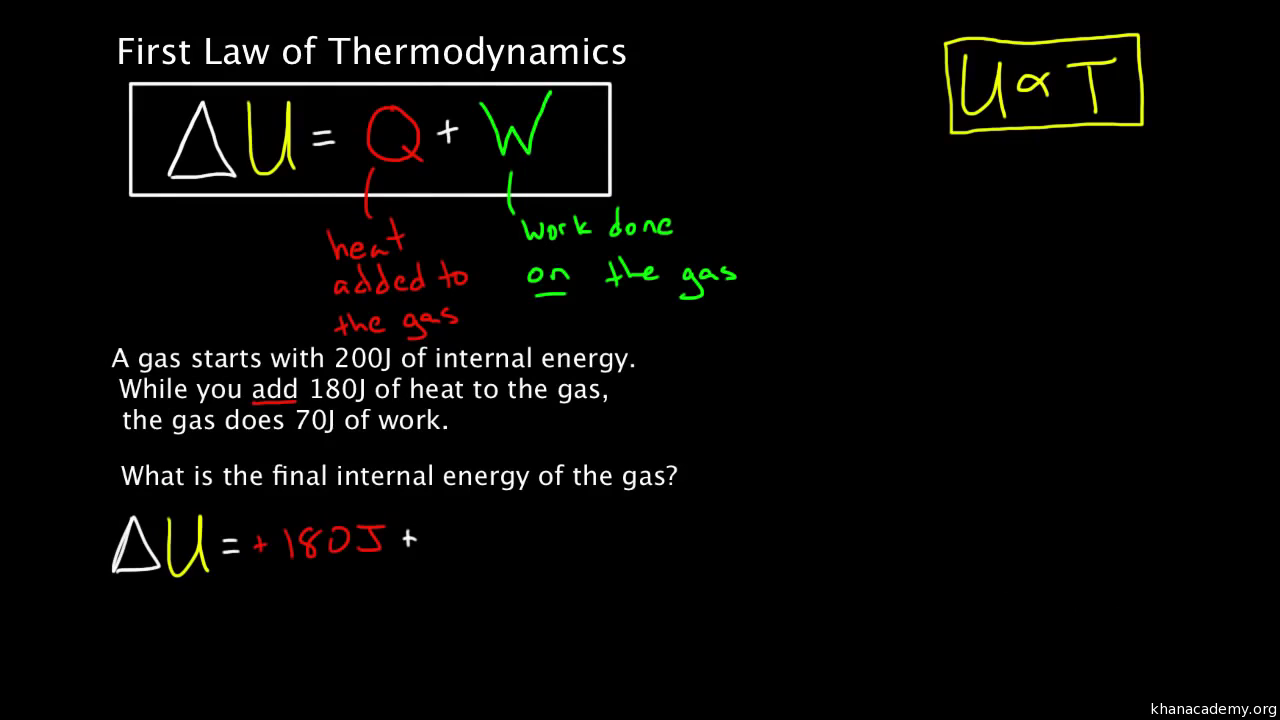
which of the following is correct equation a)△ U=△ W+△ Q b) △ U=△ Q W c) △ W= △ U+△ Q d)none of thes
a) Illustration of an armchair nanoribbon and the first Brillouin zone... | Download Scientific Diagram

Thermodynamics Thermodynamics is the study of systems involving energy in the form of heat and work. - ppt download

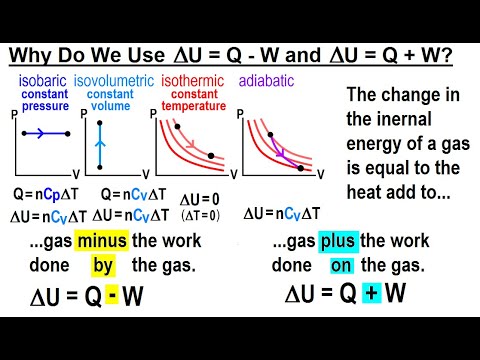
First Law of Thermodynamics | ∆U=Q-W | Mathematical Form|Thermodynamics | B.SC 2nd Year Chemistry | - YouTube