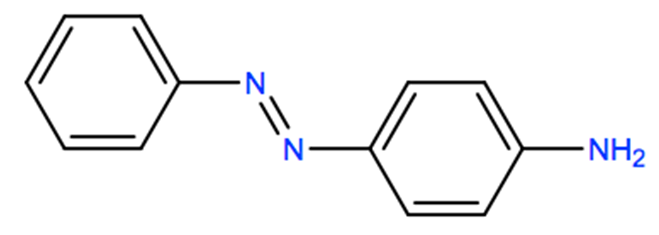
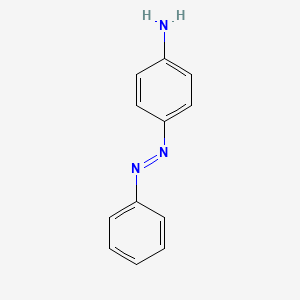
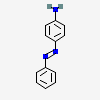
Synthesis, structure, spectral properties and DFT quantum chemical calculations of 4-aminoazobenzene dyes. Effect of intramolecular hydrogen bonding on photoisomerization - ScienceDirect
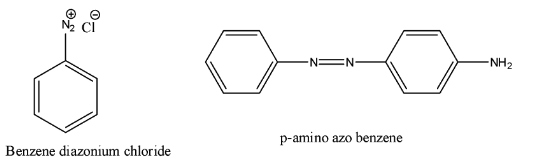
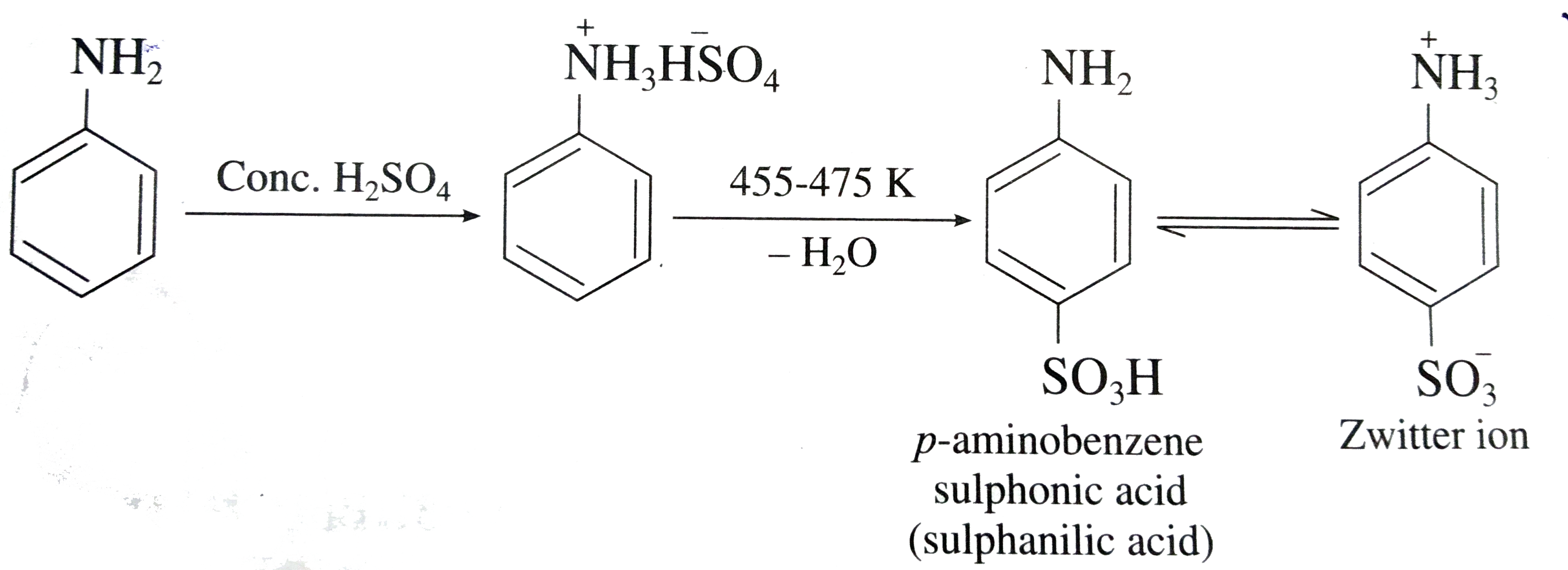
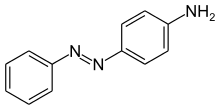
p-amino azo benzene is obtained by treating diazonium chloride with:A. PhenolB. Benzoic acidC. AlcoholD. Aniline

538-41-0 | 4,4'-Azodianiline | 4,4'-(1,2-Diazenediyl)bis-benzenamine; 4,4'-Azodi-aniline; 4,4'-Azobis-benzenamine; 4,4'-Diaminoazobenzene; NSC 17103; p,p'-Diaminoazobenzene; p-Azoaniline; p-Diaminoazobenzene; p'-Amino-p -aminoazobenzene; | C₁₂H₁₂N₄ | TRC
![104-23-4 | 4'-Aminoazobenzene-4-sulphonic Acid | 4-[2-(4-Aminophenyl)diazenyl]benzenesulfonic Acid; 4-(4-Aminophenylazo)benzenesulfonate; 4-(4-Aminophenylazo)benzenesulfonic Acid; 4-(4-Sulfophenylazo)aniline; 4-(4'-Sulfophenylazo)aniline; 4-Amino-1,1 ... 104-23-4 | 4'-Aminoazobenzene-4-sulphonic Acid | 4-[2-(4-Aminophenyl)diazenyl]benzenesulfonic Acid; 4-(4-Aminophenylazo)benzenesulfonate; 4-(4-Aminophenylazo)benzenesulfonic Acid; 4-(4-Sulfophenylazo)aniline; 4-(4'-Sulfophenylazo)aniline; 4-Amino-1,1 ...](https://www.trc-canada.com/prod-img/A577238.png)
104-23-4 | 4'-Aminoazobenzene-4-sulphonic Acid | 4-[2-(4-Aminophenyl)diazenyl]benzenesulfonic Acid; 4-(4-Aminophenylazo)benzenesulfonate; 4-(4-Aminophenylazo)benzenesulfonic Acid; 4-(4-Sulfophenylazo)aniline; 4-(4'-Sulfophenylazo)aniline; 4-Amino-1,1 ...